INTRODUCTION: CAR-T therapy is an effective treatment for hematological malignancies. However, in recent years there is a growing body of literature suggesting adverse cardiovascular (CV) events among patients treated with CAR-T therapy. Whether cardiovascular risk factors serve as determinants of CV complications and CAR-T outcomes is unclear. The aim of this study is to investigate the landscape and prognostic role of CV comorbidities in patients with non-Hodgkin lymphoma (NHL) treated with autologous CD19- CAR-T.
METHODS: We included patients with NHL treated with CD19-directed CAR-T across two tertiary care centers. Data collected included baseline demographics, CV risk factors, and outcomes. CV disease and risk factors included were: coronary artery disease (CAD), diabetes, hypertension (HTN), sleep apnea, smoking history, and stroke/ transient ischemic attack (TIA). CV events were defined as atrial fibrillation (AF), heart failure (HF), acute coronary syndrome (ACS), and cardiogenic shock. All CV events were adjudicated by a board certified cardiologist.
RESULTS: In the cohort of 345 patients, 87% had large B cell lymphoma (LBCL), 4% follicular lymphoma, and 9% mantle cell lymphoma (MCL). Predominant CV vulnerabilities included a history of smoking (41%), HTN (32%), diabetes (12%), sleep apnea (8%), history of CAD (7%), and history of stroke/TIA (5%). Patients were categorized based on number of CV risk factors or CV disease with 37% having 0, 35% with 1, and 28% with 2 or more. A total of 41 (12%) patients experienced a CV event within the first 100 days after CAR-T infusion, with 29 (8.4%) patients developing AF, 15 (4.3%) patients with HF, 2 (0.5%) patients with ACS, and 2 (0.5%) patients with cardiogenic shock. Five patients experienced more than one cardiac event.
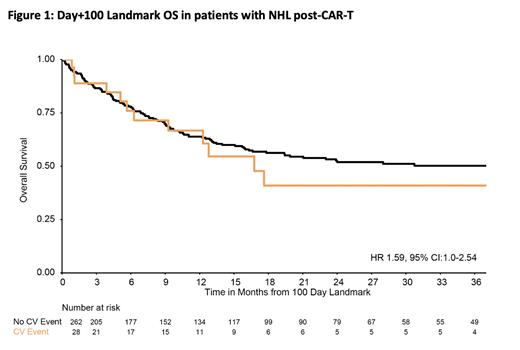
Univariable logistic regression of CV vulnerabilities revealed that patients with a history of HTN (OR 2.23, 95% CI 1.15-4.33, p = 0.018), 1 CV risk factor (OR 2.57, 95% CI 1.05-6.91) and/or 2 or more CV risk factors (OR 3.67, 95% CI 1.51-9.88) were associated with an increased risk of CV event(s) (global p=0.012). Additional baseline characteristics increasing risk of CV events included older age (OR 1.04, 95% CI: 1.01 - 1.07, p = 0.004), Karnofsky performance scale < 90 (OR 2.31, 95% CI: 1.13-5.12, p = 0.021), or stage III-IV disease (OR 2.90, 95% CI 1.11-9.94, p = 0.028). The 1-year overall survival was 62%, with a trend towards worse overall survival (HR 1.59, 95% CI:1.0-2.54, p=0.053) among patients experiencing CV events (Figure 1).
CONCLUSIONS: In this largest analysis to date, we found that patients with NHL treated with CAR-T therapy had a high burden of CV comorbidities that increases their risk of CV events. Given the incidence of CV events and trend towards decreased OS signifies the importance of cardiac evaluation and CV stratification prior to CAR-T therapy. Further investigation is essential to define strategies to mitigate adverse CV complications during treatment.
Disclosures
Giralt:Amgen, Actinuum, Celgene/BMS, Kite Pharma, Janssen, Jazz Pharmaceuticals, Johnson & Johnson, Novartis, Spectrum Pharma, Takeda: Membership on an entity's Board of Directors or advisory committees; Amgen, Actinuum, Celgene/BMS, Omeros, Johnson & Johnson, Miltenyi, Takeda: Research Funding. Palomba:Juno: Honoraria, Patents & Royalties; MustangBio: Honoraria; Kite: Honoraria; Thymofox: Honoraria; Novartis: Honoraria; Rheos: Honoraria; Seres Therapeutics: Honoraria, Patents & Royalties; Pluto Immunotherapeutics: Honoraria; Smart Immune: Honoraria; GarudaTherapeutics: Honoraria; Cellectar: Honoraria; Ceramedix: Honoraria; Synthekine: Honoraria; BMS: Honoraria. Scordo:Medscape, LLC: Honoraria; CancertNetwork (Intellisphere LLC): Honoraria; Omeros Corporation: Consultancy, Research Funding; Amgen, Inc.: Research Funding; Angiocrine Bioscience, Inc.: Research Funding. Shah:BMS: Research Funding; ArcellX: Other: DSMB; Janssen: Research Funding; Beyond Spring: Research Funding; Amgen: Research Funding. Perales:Syncopation: Honoraria; Takeda: Consultancy, Honoraria; Vor Biopharma: Consultancy, Honoraria; VectivBio AG: Consultancy, Honoraria; Medigene: Consultancy, Other; Allogene: Research Funding; Miltenyi Biotec: Honoraria; Miltenyi Biotec: Consultancy, Honoraria, Research Funding; MorphoSys: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria, Research Funding; Sellas Life Sciences: Consultancy; Cidara Therapeutics: Consultancy, Other; NexImmune: Consultancy, Current equity holder in publicly-traded company; AbbVie: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; Nektar Therapeutics: Consultancy, Honoraria, Research Funding; Allovir: Consultancy; Kite: Consultancy, Honoraria, Research Funding; Servier: Other; DSMB: Other; Celgene: Honoraria; Adicet: Honoraria; BMS: Consultancy, Honoraria; Caribou: Consultancy, Honoraria; Equillium: Consultancy, Honoraria; Exevir: Consultancy, Honoraria; Merck: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Orcabio: Consultancy, Current equity holder in publicly-traded company, Honoraria; Omeros: Consultancy, Current equity holder in publicly-traded company, Honoraria. Avigdor:Roche: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees, Other: Travel/Accommodations/Expenses; BMS: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; MSD: Research Funding.